Science and Technology Class 05
Space Debris
- Space Debris is orbital junk that is orbiting around the earth, at a very high speed.
- This debris can collide with space missions and damage satellites, space stations, and other space assets.
- The main sources of Debris are:
- (a) upper stages of rockets.
- (b) Discarded rocket stages.
- (c) Explosion of rockets or accidents in space.
- (d) Anti-satellite demonstrations.
- (e) Outdated satellites.
- There can be natural sources such as micro-meteorites
- Kessler Syndrome
- A scenario in which the density of low earth orbit is so high that one accident can produce debris, causing more accidents, and thus, enhancing the likelihood of more collisions.
- This debris is a danger to the existing missions, as well as creates challenges for future missions.
- Steps to be taken
- (a) Keeping track of larger Debris as part of space situational awareness.
- For example, Project Netra of ISRO.
- (b) Ban on anti-satellite demonstrations.
- (c) Separation of rocket stages at lower altitudes.
- (d) Dedicated missions to destroy existing pieces of debris.
- For example, the Remove Debris Mission led by the University of Surrey, and the Elsa-D mission by Astroscale.
- An international organization interagency space debris coordination committee aims to coordinate efforts at a global scale, to take care of space debris issues.
- ISRO is a member.
Nuclear Technology
- Basics of Nuclear Technology
- Nuclear Technology in Energy production
- Radioactivity
- Nuclear Technology Application: Agriculture, Medicine, Space, Industry, defence.
Basic of Nuclear Technology
- Atomic number refers to the number of protons.
- Atomic mass refers to the number of protons and neutrons.
- Isotopes are a family of elements that have the same atomic number. For example, Carbon-12 and Carbon-14, protium, deuterium, and tritium (Isotopes of Hydrogen)
- Isobars are those elements that have the same atomic mass but the same atomic number.
- The protons in the nucleus are held together by a strong nuclear force.
- This force exists between the proton-proton, neutron-neutron, and proton-neutron.
- What is Nuclear technology?
- Nuclear Technology is the application of nuclear reactions. These are the reactions in which the nucleus or nuclear particles participate.
- There are nuclear reactions, which occur in nature such as nuclear fusion in stars, and the decay of radioactive substances.
- There are nuclear reactions that can be conducted in a lab setup or an industrial setup.
- These reactions have applications in energy production, agriculture, medicine, space, etc
Nuclear Technology in Energy Production
- Energy production can occur in two ways, Nuclear Fission and Nuclear Fusion.
- Nuclear Fission
- In Nuclear fission, a heavy nucleus such as uranium-235 is bombarded with neutrons, so that a nucleus becomes unstable.
- Because of this instability, it disintegrates into lighter nuclei and produces a huge amount of energy.
- This energy can be calculated using Einstein's mass-energy equivalence equation, E= Mc^2.
- Here 'C' refers to the speed of light in a vacuum (3*10^8 m/s).
- Nuclear fission reaction produces an excess of neutrons. These neutrons can continue the fission reaction.
- The reaction will grow exponentially in an uncontrolled manner.
- Such an uncontrolled chain reaction is the mechanism, behind nuclear bombs.
- If we can absorb the excess of neutrons, the reaction becomes a controlled chain reaction, and energy output can be controlled.
- This is the mechanism behind the nuclear reactor.
Nuclear Reactors and their major components
- Nuclear reactors utilize controlled fission to produce energy.
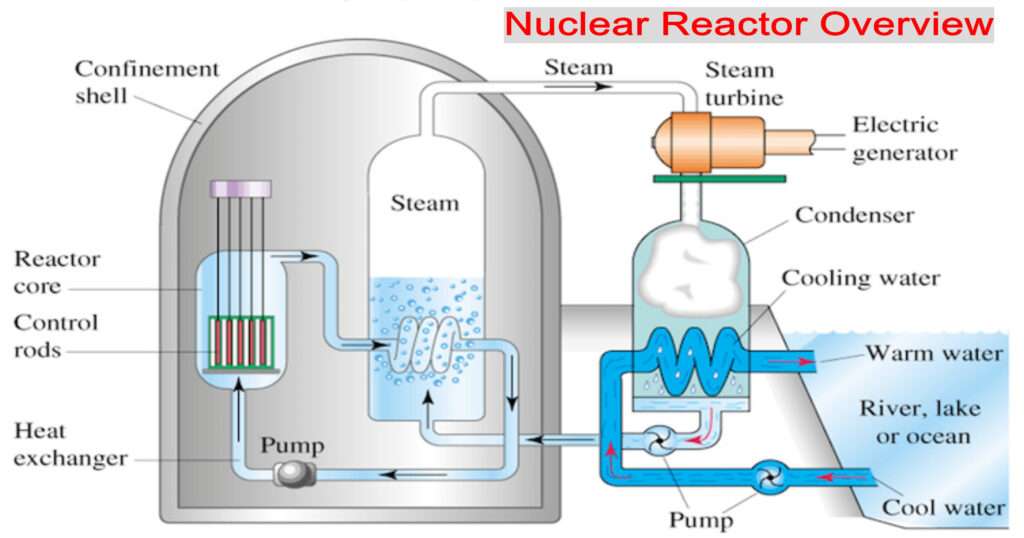
- It has the following major components:
- (a) Fissile material- Those which can undergo fission easily.
- There are two isotopes of Uranium, U-235, and U-239. Plutonium-239 is also good fissile material.
- (b) Control Rods- These are used to absorb the excess neutrons.
- These are made of Cadmium, Boron
- (c) Moderators- They are used to slow down the speed of neutrons and slower neutrons are better at causing fission.
- Water and heavy water are good moderators.
- (d) Coolant- It maintains the temperature of the core by transporting the heat away from the core of the reactor.
- Often, moderators and coolants are the same.
- For example, water and Heavy water are used as coolants.
- Liquid sodium is a good coolant, but not a good moderator.
Nuclear Fuel Cycle
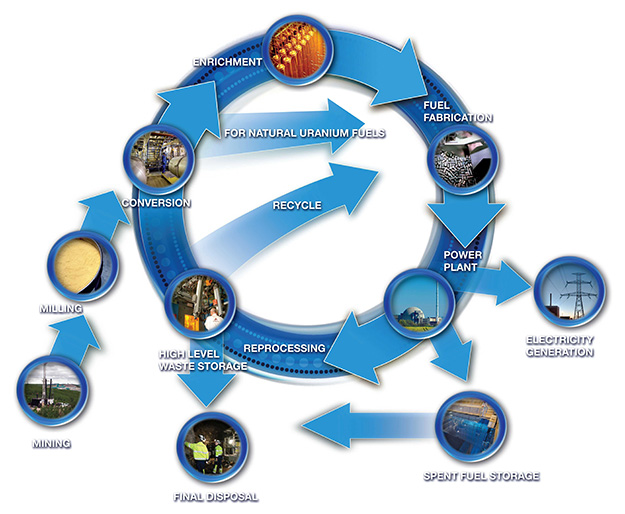
- The Nuclear Fuel Cycle signifies all the steps involved in nuclear energy production, as well as nuclear waste management.
- It comprises two parts: The front end and the back end.
- Front-end steps are the following: Mining, Milling, Enrichment, fabrication process, and Nuclear fission at the reactor.
- Mining refers to the extraction of uranium ore from a geographical location.
- Milling refers to the removal of impurities from the uranium ore by crushing and the use of physical and chemical separation methods.
- Enrichment refers to the removal of uranium 238, from the natural uranium, using isotopic separation methods.
- It is to increase the concentration of the U-235 in the ore, to 3-5 %.
- (*Natural uranium has more than 99% of uranium 238 and less than 1 per cent of uranium-235)
- In the fabrication process, enriched uranium is converted into pellets, and embedded into a tube, which makes one fuel rod.
- Many fuel rods make one assembly and many assemblies, are used in the core of the reactor.
- Depending on the capacity, the reactor can produce energy for up to 4-7 years.
- The backend step comprises of following:
- (a) Interim storage
- Nuclear waste is dangerous. It is removed from the core and is kept in a deep pool of water, for a few years.
- (b) Spent Fuel reprocessing
- We try to recover useful material, such as fissile material from nuclear waste.
- (c) Final Disposition
- Nuclear waste is deposited in the permanent underground facility.
- Some countries do not use spent fuel reprocessing, such a cycle is called the open nuclear fuel cycle.
- India's nuclear program is dependent upon spent fuel reprocessing, which is called a closed nuclear fuel cycle.
The topics for the next class: Nuclear Fission, Nuclear Fusion, Radioactivity




0 Comments